Studying The Cluster Compounds: The LNCC |ChemFam #39|
Greetings to everyone! We have already studied the 18 electron rule and hapticity. Today, we shall be studying about cluster compounds, an important topic in the organometallic chemistry. Compounds in which several metal atoms are bound together directly through M-M bonds are called metal cluster compounds. the metal toms in cluster compounds tend to agglomerate to from the maximum number of M-M bonds in such a way that MLn fragments should be sufficiently sterically unhindered to approach to within M-M bonding distance of each other.

The structures of the clusters resemble the close packed structures of the elemental metals themselves. This is due to the reason that the cluster contain unsaturated LnM fragments. The unsaturated 16 electron fragment Os(CO)4 is unstable and form a stable triangular cluster Os3(CO)12 which obeys 18 electron rule. Similarly the 15 electron fragment Rh(CO)3 forms tetrahedral cluster Rh4(CO)12 which also obeys 18 electron rule. The tendency to form M-M bond increases on descending the group in the periodic table.
The stable transition metal clusters are divided into (i) organometallic clusters and (ii) inorganic clusters.
The most extensive class of organometallic clusters belong to metal carbonyls because M(CO)n fragments are sufficiently unhindered to approach to within metal-metal bonding distance of each other. In these clusters the metals are in low oxidation states i.e., -1, 0, +1, +2.
In inorganic clusters such as Re2Cl82-, Mo2Cl84-, the metals have higher oxidation states. there are also some naked metal clusters of the post transition elements such as Sn92-.
Metal Carbonyl Clusters
The metal carbonyl clusters have CO as ligands in the metal clusters involving M-M bonds. The metal carbonyl clusters are classified into two categories :
(1) Low Nuclearity Carbonyl Clusters
(2) High Nuclearity Carbonyl Clusters
Low Nuclearity Carbonyl Clusters (LNCC)
The first metal carbonyl clusters having M-M bond is Mn2(CO)10. The low nuclearity carbonyl clusters contain metal atoms ≤4.
Calculation of number of M-M bonds using 18/16 electron rule in LNCC
The procedure for calculation of M-M bonds in a compound involves the following steps :
Step I :
Calculate total valence electron (TVE) on central metal atom in the compound say A, which is equal to the number of valence electrons of the metal plus the number of electrons from each ligand and the charge.
Step II :
Substract A from n×18 where n is the number of metals atoms in the compound. and say it is B, i.e.,
B = n×18 - A
Step III :
The value of B/2 gives the total number of M-M bonds. The value of A/n gives the number of electrons per metal.
If A/n = 18, then there will be no M-M bond
If A/n = 17, there will be one M-M bond per metal
If A/n = 16, then there will be two M-M bond
If A/n = 15, there will be three M-M bond per metal
If A/n = 14, there will be four M-M bonds per metal
This procedure can not be applied for HNCC.
Calculations of M-M bonds in some multinuclear complex is illustrated below:
(1) Mn2(CO)10
Here, A = 2 × 7 + 2 × 10 = 34
B = 2 × 18 - 34 = 2
B/2 = 2/2 = 1
∴ Total number of M-M or Mn-Mn bonds = 1
A/n = 34/2 = 17
∴ There is one Mn-Mn bond per metal.
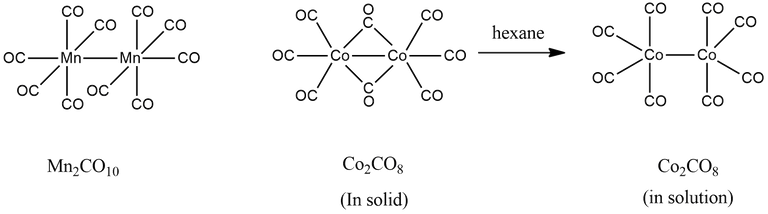
(2) Co2(CO)8
Here, A = 2 × 9 + 2 × 8 = 34
B = 2 × 18 - 34 = 2
B/2 = 2/2 = 1
∴ Total number of M-M or Co-Co bonds = 1
A/n = 34/2 = 17
∴ There is one Co-Co bond per metal.
(3) Fe2(CO)9
Here, A = 8 × 2 + 2 × 9 = 34
B = 2 × 18 - 34 = 2
B/2 = 2/2 = 1
∴ Total number of M-M or Fe-Fe bonds = 1
A/n = 34/2 = 17
∴ There is one Fe-Fe bond per metal.
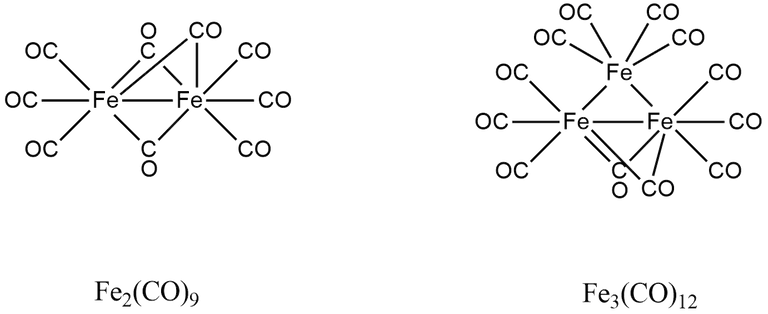
(4) Fe3(CO)12
Here, A = 3 × 8 + 12 × 2 = 48
B = 3 × 18 - 48 = 2
B/2 = 6/2 = 3
∴ Total number of M-M or Fe-Fe bonds = 3
A/n = 48/3 = 16
∴ There is o two Fe-Fe bond per metal.
(5) Rh4(CO)12
Here, A = 4 × 9 + 12 × 2 = 60
B = 4 × 18 - 60 = 2
B/2 = 12/2 = 6
∴ Total number of M-M or Rh-Rh bonds = 6
A/n = 60/2 = 15
∴ There is three Rh-Rh bond per metal.
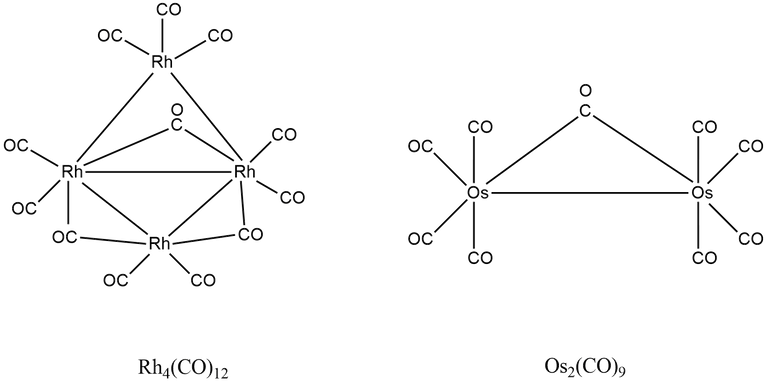
(6) Os2(CO)9
Here, A = 2 × 8 + 9 × 2 = 34
B = 2 × 18 - 34 = 2
B/2 = 2/2 = 1
∴ Total number of M-M or Os-Os bonds = 1
A/n = 34/2 = 17
∴ There is one Os-Os bond per metal.
(7) Ru3(CO)12
Here, A = 3 × 8 + 12 × 2 = 48
B = 3 × 18 - 48 = 6
B/2 = 6/2 = 3
∴ Total number of M-M or Ru-Ru bonds = 3
A/n = 48/3 = 16
∴ There is two Ru-Ru bond per metal.
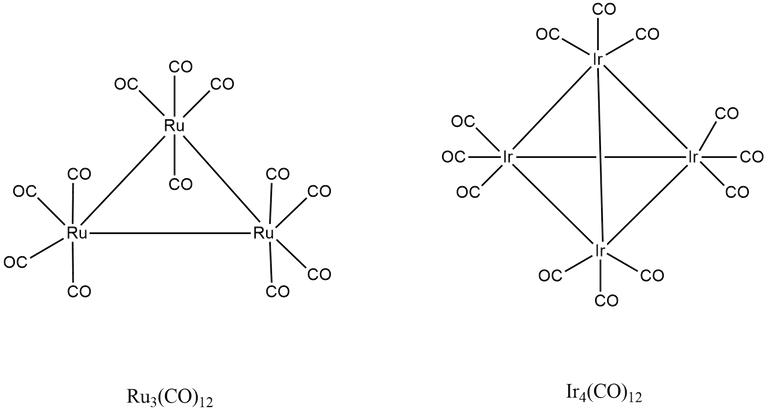
(8) Ir4(CO)12
Here, A = 4 × 9 + 12 × 2 = 60
B = 4 × 18 - 60 = 2
B/2 = 12/2 = 6
∴ Total number of M-M or Ir-Ir bonds = 6
A/n = 60/2 = 15
∴ There is three Ir-Ir bond per metal.
The 18 electron rule is of great importance for predicting the number of M-M bonds, but it does not play any role to distinguish the bridging and terminal ligands. The factors causing the CO ligands to bridge or not bridge are the steric factors. V(CO)6 does not dimerise as it would become seven coordinate and increase in steric hinderance. In Mn2(CO)10, if bridging occurs, there will be increase in coordination number beyond six and therefore it will be destabilized.
So, this is how one could predict the number of M-M bonds in a carbonyl cluster compound, which are crucial for studying the organometallic compounds. I shall discuss the HNCC later. Till then happy learning!
Organometallic Chemistry (Evans)
Organometallic compounds- An overview
Biochemistry of Calcium: Role of Calcium in Muscle Contraction |ChemFam #38|
Biosynthesis of Fatty Acids: De Novo Synthesis of Fatty Acids |ChemFam #37|
Hapticity and The Eighteen Electron Rule |ChemFam #36|
An Introduction To Organometallic Chemistry |ChemFam #35|
Applications of Zeolites: The 3D Molecular Sieves |ChemFam #34|
Properties of Zeolites: The 3D Molecular Sieves |ChemFam #33|
Zeolites: The 3D Molecular Sieves |ChemFam #32|
The Beauty of Eucalyptus Tree |ChemFam #31|
The Accidental Cure for Cancer: Cisplatin |ChemFam #29|
Acceptorless Dehydrogenation and Related Transformations |ChemFam #28|
PS The thumbnail image is being created by me using canva.com


Congratulations @splash-of-angs63! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 80 posts.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out our last posts:
Wow I'm impressed how you draw formulas here 🤩 once chemistry was my favorite subject but than i failed to learn about it's deep mechanism 😕
I use a software called chemdraw to draw the structures. Thank you for stopping by 🤗
Ohhh i see that'd a nice way
This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.