Chemistry of Glass "Part 2": Formation of a vitreous network.
Glass is made up of three oxides: sodium oxide Na₂O, lime CaO, and silica SiO₂. The three primary components for making glass—limestone CaCO₃, sand, and soda Na₂CO₃— are where these oxides are derived. In order to create sodium and calcium silicates, these three elements must first be combined and powdered before being heated to a temperature exceeding 1260 °C, at which point they melt and emit carbon dioxide gas. In addition to not crystallizing even when cooled below its melting point, the produced compound has a low melting point and a high viscosity. At normal temperature, it becomes hard as a crystalline solid.
For an oxide to create a vitreous network, William Holder Zachariasen identified four conditions:
1- The formative atom A has the coordinates (3, 4). Therefore, only a few anions (O2-) may surround cations. Example: AO3, SiO4, AO4.
2- More than two cations cannot be bonded to an oxygen at once: Si_O_Si.
3- Tetrahedra and octahedra have a vertex but never faces in common with other polyhedra.
4- To create a 3D network, each polyhedron must have at least three vertices in common with its neighbors.
The following oxides satisfy these requirements:
- SiO2, B2O3 (Boric anhydride).
- As2O3 (Arsenic oxide).
- GeO3 (Germanium oxide).
- P2O5 (Phosphorus oxide).
Notes:
Bridging oxygen is an oxygen that is attached to a silicon (Si-O-Si) on both of its 02 sides. With more Si-O-Si bridges, the vitreous network becomes more powerful.
The vitreous structure becomes weaker as a result of the addition of Na2O due to bond breaking.
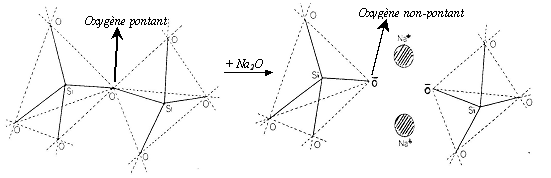
Breaking of a Si-O-Si bridge by addition of a Na2O network modifier molecule in an oxide glassCaO entry also causes network discontinuities and breakdowns.
Alkali cations and alkaline earth cations are glass network modifiers.
With a typical coordination of 6, Aluminium is a lattice modifier. Aluminium may form AlO4 under specific circumstances when it has a coordination of 4.
References:
- [General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
- Glass and Ceramic Technology Course (2021). Professor Khelifa- Department of Materials Process Engineering- University of Mostaganem. Algeria.
- Les matériaux au cœur du processus d'innovation- Clefs CEA No 59.
- Neumann, Florin. "Glass: Liquid or Solid – Science vs. an Urban Legend". Archived from the original on 9 April 2007. Retrieved 8 April 2007.
- Helène Tregouët. Structure et cristallisation de verres d’oxydes simples riches en bore et en terres rares. Chimie-Physique [physics.chem-ph]. Université Pierre et Marie Curie- Paris VI, 2016. Français. NNT: 2016PA066032. tel-01358710.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.