@PROOFOFBRAIN: CORROSION AND IT PREVENTIVE MEASURE
Today I was going through my project work which I’ll be defending later this week so felt I should share this with us so that we can all learn from it.
So, the topic is corrosion inhibition of metals.
I believe we all know what a metal is. So let’s take a look the picture below.

Image source: european-coatings.com
As we can see from the above picture, we have a corroded and a non corroded pipe.
So, a metal will corrode when it is expose to the atmosphere and comes in contact with moisture and oxygen.
Corrosion has been a major problem in construction, chemical industry, oil and gas industry, food industry and so on. Corrosion also affects many of our home appliances.
Below are examples of home appliances which has been destroy as a result of corrosion.




DISADVANTAGES OF CORROSION
- When our home appliances are destroyed as a result of corrosion, there will be need for replace of such item.
- Also in the manufacturing industries, when corrosion affects the machine used for production, there will be need for replacement which will lead to increase in cost of production and also increase in price of the products.
- In oil and gas industries, pipes that are used to convey petroleum from one place to another can be affected by corrosion, and when such pipe is affected by corrosion, there will the reduction in thickness of the pipe which will eventually leads to leakage of such pipe.
- Corrosion is one of the major problem faced by chemical industries.
Naturally, when chemical comes in contact with metals, the metal will corrode. - Corrosion also affect structures such as bridge, and a corroded bridge can collapse at any time which can cause serious injury to people and even lead to loss of lives and properties.
So, corrosion cannot be totally eradicated but it can be prevented by various methods.
These methods include: painting, greasing, electroplating of the metals and do on.
But the methods mentioned above are not efficient, that’s why we have a lots of scientists carrying out research on how corrosion can be prevented.
So, the use of organic and inorganic compounds has been the most effective ways of preventing corrosion. But during the course of my research, I discovered that Organics compounds are more preferable. The reason why Organic compounds are more preferable is that, organic compounds are less toxic and easy to work with compare to inorganic compounds that are more toxic.
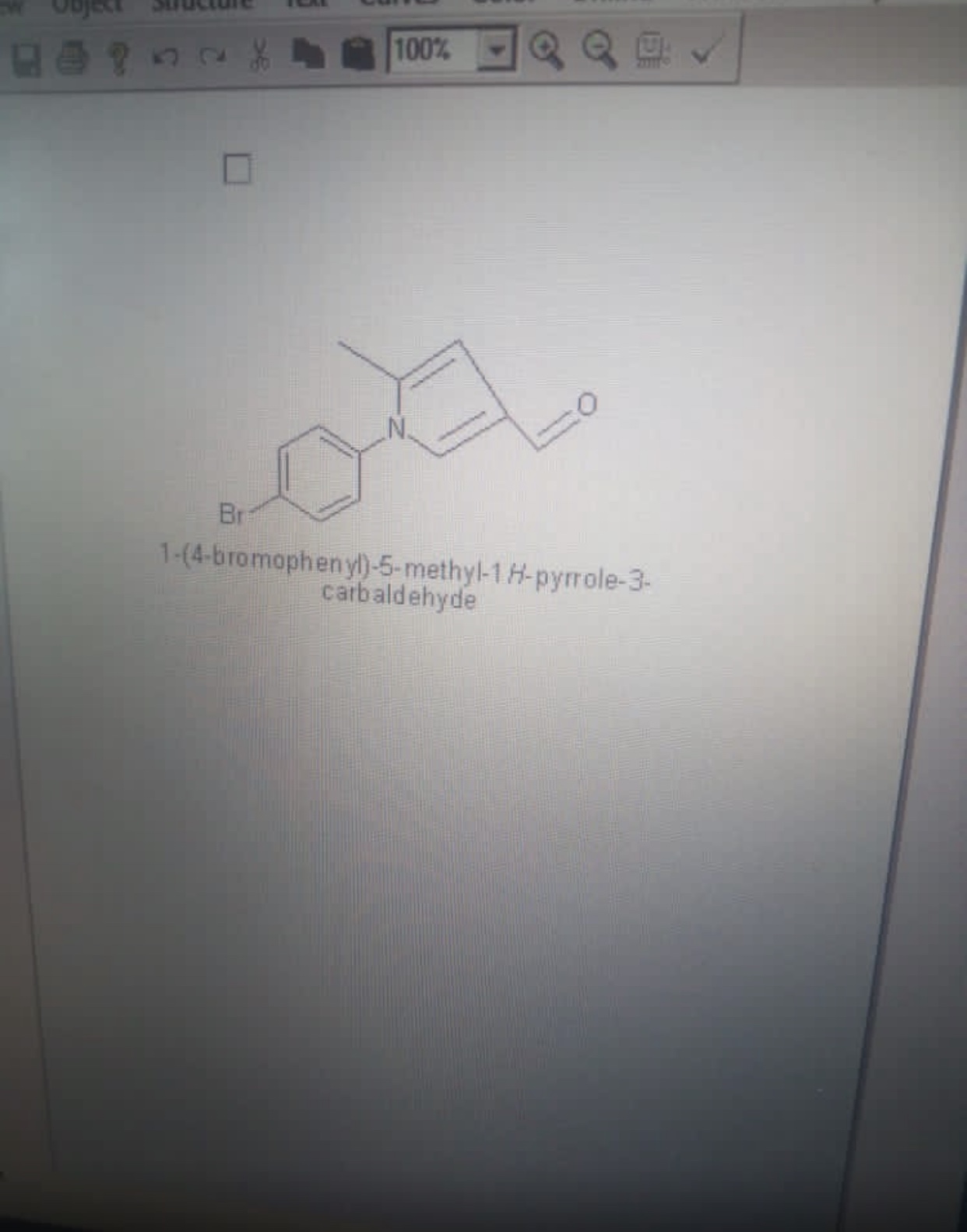
For an organic compound to be qualify as a corrosion inhibitor, it must possess so characteristic, the characteristics include:
- It must contain at least one of the following element: sulphur, oxygen, nitrogen
- It must be conjugated I.e it must have alternating single bonds and double bonds
- It must be a cyclic compound
For this reason, I decided to use the compound in the picture below as a corrosion inhibitor because it possess the above characteristics.
Thank you.